15 September 2016
WASHINGTON, DC — A great algae bloom at the bottom of the world is teaching scientists more about how an iconic symbol of the United Kingdom came to be.

The White Cliffs of Dover have been a symbol of England at least since Roman times. New research is teaching scientists more about how this great structure came to be.
Credit: Immanuel Giel, CC by 3.0 via Wikimedia Commons.
The White Cliffs of Dover span England’s southeastern coastline for 16 kilometers (10 miles) and reach as tall as 110 meters (350 feet) high. Facing the narrowest part of the English Channel, the cliffs have come to symbolize England since the time of Julius Caesar, often the first and last view travelers have of the country by sea.
The sheer cliffs are composed of white chalk, or calcite, made by coccolithophores – tiny, single-celled algae at the bottom of the marine food chain. Coccolithophores build hard, saucer-shaped calcite plates around themselves that sink and accumulate on the sea floor when the algae die, compacting and hardening into chalk. The White Cliffs’ chalk was laid down in a shallow sea above present-day England almost 100 million years ago and thrust upward by movements of the Earth’s crust.
Now, researchers outline in a new study the ocean conditions necessary for coccolithophores to flourish, conditions that likely allowed the White Cliffs to form nearly 100 million years ago. The new information comes from an unlikely source: a great bloom of coccolithophores in the Southern Ocean known as the Great Calcite Belt.
The new study, published in Global Biogeochemical Cycles, a journal of the American Geophysical Union, describes why the Great Calcite Belt exists and also clarifies coccolithophores’ role in the global carbon cycle.
The algae sequester carbon into their calcite plates but that process also increases concentrations of carbon dioxide in ocean water, according to William Balch, a biological oceanographer at Bigelow Laboratory for Ocean Sciences in East Boothbay, Maine, and lead author of the new study.
“The Great Calcite Belt is significant because this gigantic area of the ocean is full of these organisms that are fixing carbon,” Balch said.
Microscopic mirrors
Every year during the southern hemisphere’s summer, a ring of bright, reflective water encircles Antarctica. In 2011, Balch and his colleagues reported the highly reflective water was associated with a bloom of coccolithophores.
The algae act as microscopic mirrors: their calcite plates reflect light, brightening the ocean. The band of bright water in the Southern Ocean became known as the Great Calcite Belt.

In a coccolithophore bloom, like this one in the Barents Sea, water turns brilliant shades of blue and green.
Credit: Jeff Schmaltz/NASA Earth Observatory.
“If you take the Earth and look at it upside down, it looks like a bullseye,” said Marlon Lewis, an oceanographer at Dalhousie University in Halifax, Nova Scotia who was not involved with the study. “But we didn’t know what it was and [Balch’s 2011 study] kind of nailed it.”
For the new study, Balch and his colleagues took two research cruises to the Southern Ocean in 2011 and 2012. They examined the belt’s water to determine what species of coccolithophores were present, how abundant they were and how they manage to outcompete other types of algae, such as large diatoms, in this area of ocean.
They found coccolithophores depend on concentrations of three key nutrients: nitrate, silicate, and iron. Diatoms need silicate to build glassy shells around themselves, so in areas where silicate was more abundant than nitrate, diatoms outcompeted coccolithophores. Coccolithophores, on the other hand, flourished where nitrate was more abundant than silicate. In these areas there was also enough iron for coccolithophores to thrive, but not enough for diatoms. Coccolithophores also grew better than most diatoms in low-iron regions, according to Balch.
Coccolithophores also flourish where different water masses diverge. At these boundaries, upwelling of deep water brings to the surface trace metals and nutrients coccolithophores need to survive, Balch said.
“These regions can be oases of fertilizer coming up to the surface for these plants,” he said.
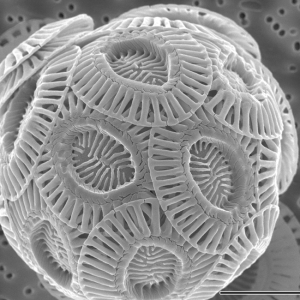
This microscopic view of a coccolithophore shows the saucer-shaped calcite plates the algae build around themselves. Scientists suspect the plates help coccolithophores survive and evade predators.
Credit: Alison R. Taylor/University of North Carolina Wilmington Microscopy Facility, CC BY 2.5 via Wikimedia Commons.
The researchers also examined the role of coccolithophores in sequestering carbon. They found diatoms send more organic carbon to the deep ocean than coccolithophores do, but coccolithophores sequester carbon more efficiently.
Unexpectedly, formation of coccolithophores’ calcite plates releases carbon dioxide into the surrounding ocean water, according to the study. This effect on water chemistry may be more important to the global carbon cycle than their role in sending carbon more efficiently to the depths, Balch said.
A White Cliffs in the making?
The new study lays out the surface ocean conditions necessary for high concentrations of calcite plates from coccolithophores to form and sink to the sea floor, much as they did nearly 100 million years ago to form the White Cliffs of Dover.
But it is difficult to say whether the plates from the Great Calcite Belt will form chalk and produce a large structure like the Dover cliffs, according to Lewis. To build a Dover-like structure, many layers of calcite would need to be deposited on the seafloor over millions of years, he said.
According to Balch, there’s no guarantee this will happen, but a recent paper in the journal Geology shows calcite-rich sediments have been found directly under the Great Calcite Belt.
“While we don’t have the great cliffs of the Southern Ocean, there is solid evidence that the calcite is making it to the sea floor,” he said.
###
The American Geophysical Union is dedicated to advancing the Earth and space sciences for the benefit of humanity through its scholarly publications, conferences, and outreach programs. AGU is a not-for-profit, professional, scientific organization representing more than 60,000 members in 139 countries. Join the conversation on Facebook, Twitter, YouTube, and our other social media channels.
Notes for Journalists
This research article is open access. A PDF copy of the article can be downloaded at the following link: http://onlinelibrary.wiley.com/doi/10.1002/2016GB005414/pdf
Journalists and PIOs may also order a copy of the final paper by emailing a request to Lauren Lipuma at [email protected].
Please provide your name, the name of your publication, and your phone number.
Neither the paper nor this press release is under embargo.
“Factors regulating the Great Calcite Belt in the Southern Ocean and its biogeochemical significance”
Authors:
William M. Balch, Benjamin S. Twining, Bruce C. Bowler, Dave T. Drapeau, Laura C. Lubelczyk, Catherine Mitchell, Sara Rauschenberg: Bigelow Laboratory for Ocean Sciences, East Boothbay, Maine, U.S.A.;
Nicholas R. Bates: Bermuda Institute of Ocean Sciences (BIOS), Inc., St. George, Bermuda, and Department of Ocean and Earth Sciences, University of Southampton, Southampton, U.K.;
Phoebe J. Lam: Department of Marine Chemistry and Geochemistry, Woods Hole Oceanographic Institution, Woods Hole, Massachusetts, U.S.A., now at Department of Ocean Sciences, University of California, Santa Cruz, California, U.S.A.;
Sarah Z. Rosengard: Department of Marine Chemistry and Geochemistry, Woods Hole Oceanographic Institution, Woods Hole, Massachusetts, U.S.A., and Massachusetts Institute of Technology, Cambridge, Massachusetts, U.S.A.;
Rebecca Garley: Bermuda Institute of Ocean Sciences (BIOS), Inc., St. George, Bermuda.
Contact Information for the Authors:
William M. Balch: +1 (207) 315-2567 x301, [email protected]
Lauren Lipuma
+1 (202) 777-7396
[email protected]
Bigelow Laboratory for Ocean Sciences Contact:
Steven Profaizer
+1 (207) 315-2567 x103
[email protected]